Concept Of Energy Profile In Organic Chemistry
The equation below shows an organic chemistry reaction in which a bromine atom is being replaced by an OH group in an organic compound. In this post we are going to talk about a trick or key concept.
 E1 Energy Diagram Transition State Forming A Double Bond Energy Activities Reactions Chemistry
E1 Energy Diagram Transition State Forming A Double Bond Energy Activities Reactions Chemistry
If you are starting to face basic organic chemistry you may feel overwhelmed with the huge amount of material.

Concept of energy profile in organic chemistry. Energy profiles for reactions which go via a single transition state only. Free energy of activation G decreases rate constant k increases catalysis does not affect the end point of an equilibrium but only accelerates how quickly equilibrium is attained free energy of the reaction G remains unchanged equilibrium constant K eq remains unchanged 5 Energy profile. The heat of hydrogenation of cyclohexene 286 kcalmole.
Explain the concept of free rotation about a carbon-carbon single bond. Enthalpy change H is the amount of energy absorbed or released by a chemical reaction. Conformations of Organic Molecules Conformational Analysis Organic molecules can assume different spatial arrangements conformations which are gener-ated by rotation about single bonds.
This is much easier to talk about with a real example. This is possible only in conjugated systems or molecules having. Practical Topics Green chemistry can be used to illustrate many fundamental chemistry principles throughout the.
It is the height of the potential energy barrier between the potential energy minima of the reactants and products. Increasing the efficiency and reducing energy requirements of synthesis isolation and purification operations can reduce the environmental impact of a process. The experimental findings as well as the results of theoretical analysis by means of molecular mechanics and ab initio calculations have contributed decisively to our present state of knowledge of the structure energy and reactivity of organic compounds.
C 6 H 12 O 6 s 6O 2 g 6CO 2 g 6H 2 O 670 kcal. To relate the concept of energy change to chemical reactions that occur in the body. After completing this section you should be able to.
An important reaction that provides energy for our bodies is the oxidation of glucose C 6 H 12 O 6. Even so the chemical reactions found in the human body follow the same principles of energy that other chemical reactions follow. The starting compound is bromoethane and the organic product is ethanol.
On an energy profile the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products. If it takes 1 energy unit to warm 025 g of water by 1C then it takes over 15100 energy units to put that 025 g of water into earth orbit. For example the estimated resonance energy of benzene from the heat of hydrogenation data is 36 kcalmole which can be shown in the following way.
Basic concepts of organic chemistry ELECTRONEGATIVITY A covalent bond where the electrons are shared equally is called a nonpolar bond eg HH and an unequal sharing of the pair of bonding electrons results in a polar bond. The concept of resonance energy in elementary organic chemistry Journal of Chemical Education. Resonance Energy of Benzene Energy of cyclohexatriene most stable canonical structure Energy of Benzene Actual Molecule.
The term activation energy was introduced by the Swedish scientist Svante Arrhenius in 1889. The author describes an empirically-based presentation of resonance energy that is perfectly within reach of introductory organic students. The representation of a molecule by more than one canonical structures is known as resonance.
The chemistry of the human body or any living organism is very complex. Conformational analysis is an im-. The lowest energy conformation of ethane shown in the figure above is called the staggered conformation in which all of.
Considering that 1 mol of C 6 H 12 O 6 s has a volume of about 115 mL we can see that glucose is a compact source of energy. A detailed analysis of the various conformations adopted by individual molecules is termed Conformational Analysis. Introductory organic chemistry courses can seem like the most difficult ones.
An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Activation energy is denoted by E a and typically has units of kilojoules per mole kJmol or kilocalories per mole kcalmol. Introduction to Energy and Chemistry It takes energy to launch a spaceship into space.
An Introduction to Organic Compounds.
 Energy Profile Diagram Google Sogning Organic Chemistry Chemical Reactions Chemistry
Energy Profile Diagram Google Sogning Organic Chemistry Chemical Reactions Chemistry
 Organic Chemistry Energy Diagrams Organic Chemistry Chemistry Physical Chemistry
Organic Chemistry Energy Diagrams Organic Chemistry Chemistry Physical Chemistry
 Reaction Profiles Potential Energy Chemistry Profile
Reaction Profiles Potential Energy Chemistry Profile
 Electrophilic Aromatic Substitution Energy Diagram Organic Chemistry Chemistry Organic Chemistry Notes
Electrophilic Aromatic Substitution Energy Diagram Organic Chemistry Chemistry Organic Chemistry Notes
 Sn1 Energy Diagram Transition States Activation Energy Intermediates Energy Activities Reactions Organic Chemistry
Sn1 Energy Diagram Transition States Activation Energy Intermediates Energy Activities Reactions Organic Chemistry
 Exothermic Reaction Wikipedia Exothermic Reaction Energy Chemistry
Exothermic Reaction Wikipedia Exothermic Reaction Energy Chemistry
 Free Energy And Equilibrium Biological Chemistry Chemistry Basics Teaching Chemistry
Free Energy And Equilibrium Biological Chemistry Chemistry Basics Teaching Chemistry
 Activation Energy Chemistry Chemistry Experiments Energy Activities
Activation Energy Chemistry Chemistry Experiments Energy Activities
 Transition State Theory Theories Organic Chemistry Chemistry
Transition State Theory Theories Organic Chemistry Chemistry
 Energy Diagrams Sn1 And Sn2 Chemistry Help Potential Energy Organic Chemistry
Energy Diagrams Sn1 And Sn2 Chemistry Help Potential Energy Organic Chemistry
 Heat And Chemical Reactions Energie
Heat And Chemical Reactions Energie
 Endo And Exo Diels Alder Energy Diagram Endo Chemistry Organic Chemistry
Endo And Exo Diels Alder Energy Diagram Endo Chemistry Organic Chemistry
 Energy Profile Chemistry Chemistry Energy Organic Chemistry
Energy Profile Chemistry Chemistry Energy Organic Chemistry
 Energy Profile Diagrams Endothermic Exothermic Reactions 3 Exothermic Reaction Chemical Energy Energy
Energy Profile Diagrams Endothermic Exothermic Reactions 3 Exothermic Reaction Chemical Energy Energy
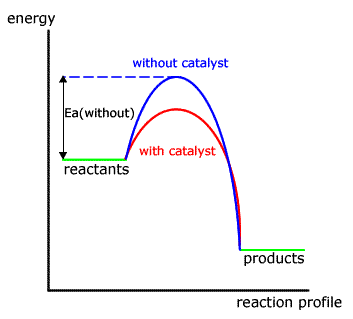
 Energy Profile Of Catalyzed And Uncatalyzed Reactions Mcat Study Biochemistry Study Tools
Energy Profile Of Catalyzed And Uncatalyzed Reactions Mcat Study Biochemistry Study Tools
 H Is A State Function Because E P V Are State Functions So It Depends Only On The Difference Betwee Teaching Chemistry Chemistry Education Science Chemistry
H Is A State Function Because E P V Are State Functions So It Depends Only On The Difference Betwee Teaching Chemistry Chemistry Education Science Chemistry
 Enzymes Chemical Energy Energy Activities Enzymes
Enzymes Chemical Energy Energy Activities Enzymes
 A Generalised Energy Profile Diagram For Kinetic Versus Thermodynamic Product Reaction Organic Chemistry Chemistry Physical Chemistry
A Generalised Energy Profile Diagram For Kinetic Versus Thermodynamic Product Reaction Organic Chemistry Chemistry Physical Chemistry

Post a Comment for "Concept Of Energy Profile In Organic Chemistry"