Definition Of Free Energy In Chemistry
The definition of free energy is where T is the temperature in Kelvin. Free energy has the dimensions of energy and its value is determined by the state of the system and not by its history.
 Gibbs Free Energy Free Energy Science Blog Energy
Gibbs Free Energy Free Energy Science Blog Energy
Gibbs free energy is a thermodynamics property defined as G H TS.

Definition of free energy in chemistry. For studying chemical reactions the relationship involves changes in the three thermodynamic quantities. The units of energy are joules or ergs. The change in free energy G is equal to the sum of the enthalpy plus the product of the temperature and entropy of the system.
G H - TS Where G is the Gibbs free energy H is. Gibbs free energy is the energy that may be converted into work in a system that is at constant temperature and pressure. Khan Academy is a 501c3 nonprofit organization.
Gibbs free energy denoted G combines enthalpy and entropy into a single value. In physics and physical chemistry free energy refers to the amount of internal energy of a thermodynamic system that is available to perform work. It is defined by the equation.
Free energy is energy that is available to do work. Annual Reports in Computational Chemistry 2005. Spontaneous reactions release free energy as they proceed.
Many chemical reactions and physical processes release energy that can be used to do other things. There are different forms of thermodynamic free energy. Free energy or Gibbs free energy G is the energy available in a system to do useful work and is different from the total energy change of a chemical reaction.
Free energies are central quantities to both thermodynamics and kinetics relating to experimentally determined properties such as equilibrium constants and reaction rates. Free energy in thermodynamics energy-like property or state function of a system in thermodynamic equilibrium. Free energy is a measure of the capacity of the system to do work.
Gibbs Free Energy. An analogy for Gibbs free energy Our mission is to provide a free world-class education to anyone anywhere. Free energy is used to determine how systems change and how much work they can produce.
Free energy A thermodynamic quantity that is the difference between the internal energy of a system and the product of its absolute temperature and entropy. It is a thermodynamic property that was defined in 1876 by Josiah Willard Gibbs to predict whether a process will occur spontaneously at constant temperature and pressure. In thermodynamics the Helmholtz free energy is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature and volume.
The Gibbs free energy is a chemical potential energy in a substance. The negative of the change in the Helmholtz energy during a process is equal to the maximum amount of work that the system can perform in a thermodynamic process in which volume is held constant. A cardinal thermodynamic principle is that systems change toward minimum free energy.
G can predict the direction of the chemical reaction under two conditions. A thermodynamic quantity equivalent to the capacity of a system to do work. If its value is negative the system will have a tendency to do work spontaneously as in an exothermic chemical reaction.
Entropies must be multiplied by temperature to get units of energy. Gibbs free energy is a measure of the potential for reversible or maximum work that may be done by a system at constant temperature and pressure. Nuclear energy energy that can be liberated by changes in the nucleus of an atom as by fission of a heavy nucleus or by fusion of light nuclei into heavier ones with accompanying loss of mass.
Since H and TS are energy terms G is also energy terms further specific heat or enthalpy of reaction temperature and entropy are all state functions and perfectly differential quantitiesTherefore G also a state function and perfectly differential quantity. Energy - physics a thermodynamic quantity equivalent to the capacity of a physical system to do work. Definition of Gibbs Free Energy.
When the fuel in a car is burned some of the released energy is used to power the vehicle. See also Gibbs free energy The origin of the cell voltage is then the difference in free energy between Li ions in the crystal structures of the two electrode materials. Free energy Gibbs free energy G the energy equal to the maximum amount of work that can be obtained from a process occurring under conditions of fixed temperature and pressure.
Energy can take a wide variety of forms free energy natural philosophy physics - the science of matter and energy and.
 Entropy Free Energy And Thermodynamic Equilibrium Chemistry Net Free Energy Entropy Energy
Entropy Free Energy And Thermodynamic Equilibrium Chemistry Net Free Energy Entropy Energy
 What Is Chemical Energy Definition And Examples Chemical Energy Energy Science Activities Energy Transformations
What Is Chemical Energy Definition And Examples Chemical Energy Energy Science Activities Energy Transformations
 Free Energy And Equilibrium Chemistry Basics Biological Chemistry Teaching Chemistry
Free Energy And Equilibrium Chemistry Basics Biological Chemistry Teaching Chemistry
 Energy Definition And Types Physics Education Quotes For Teachers Online Tutoring
Energy Definition And Types Physics Education Quotes For Teachers Online Tutoring
 Difference Between Enthalpy And Internal Energy Definition Units Formula For Calculation Properti Internal Energy Potential Energy Organic Chemistry Study
Difference Between Enthalpy And Internal Energy Definition Units Formula For Calculation Properti Internal Energy Potential Energy Organic Chemistry Study
 The Second Law Of Thermodynamics Second Law Of Thermodynamics Thermodynamics Physics And Mathematics
The Second Law Of Thermodynamics Second Law Of Thermodynamics Thermodynamics Physics And Mathematics
 Gibbs Free Energy Equilibrium Constant Enthalpy Entropy Equations Practice Problems Youtube Free Energy Free Energy Projects Energy Technology
Gibbs Free Energy Equilibrium Constant Enthalpy Entropy Equations Practice Problems Youtube Free Energy Free Energy Projects Energy Technology
 Electrode Potential Energy Definition Formula And Example Potential Energy Chemistry Notes Electrodes
Electrode Potential Energy Definition Formula And Example Potential Energy Chemistry Notes Electrodes
 Difference Between Endergonic And Exergonic Free Energy Energy Technology Thermodynamics
Difference Between Endergonic And Exergonic Free Energy Energy Technology Thermodynamics
 Reactions In Which Energy Is Released Are Exothermic Reactions And Those That Take In Heat Energy Are Exothermic Reaction Chemistry Lessons Teaching Chemistry
Reactions In Which Energy Is Released Are Exothermic Reactions And Those That Take In Heat Energy Are Exothermic Reaction Chemistry Lessons Teaching Chemistry
 Forms Of Energy Kidspressmagazine Com Thermal Energy Chemical Energy Energy Projects
Forms Of Energy Kidspressmagazine Com Thermal Energy Chemical Energy Energy Projects
 Completely Editable This 25 Slide Powerpoint Presentation Covers In Detail All Of The Following Ap Chemistry Concepts Thermodynamics Chemistry Ap Chemistry
Completely Editable This 25 Slide Powerpoint Presentation Covers In Detail All Of The Following Ap Chemistry Concepts Thermodynamics Chemistry Ap Chemistry
 Unit 3 Elaboration Enthalpy Entropy And Free Energy Free Energy Energy Calculus
Unit 3 Elaboration Enthalpy Entropy And Free Energy Free Energy Energy Calculus
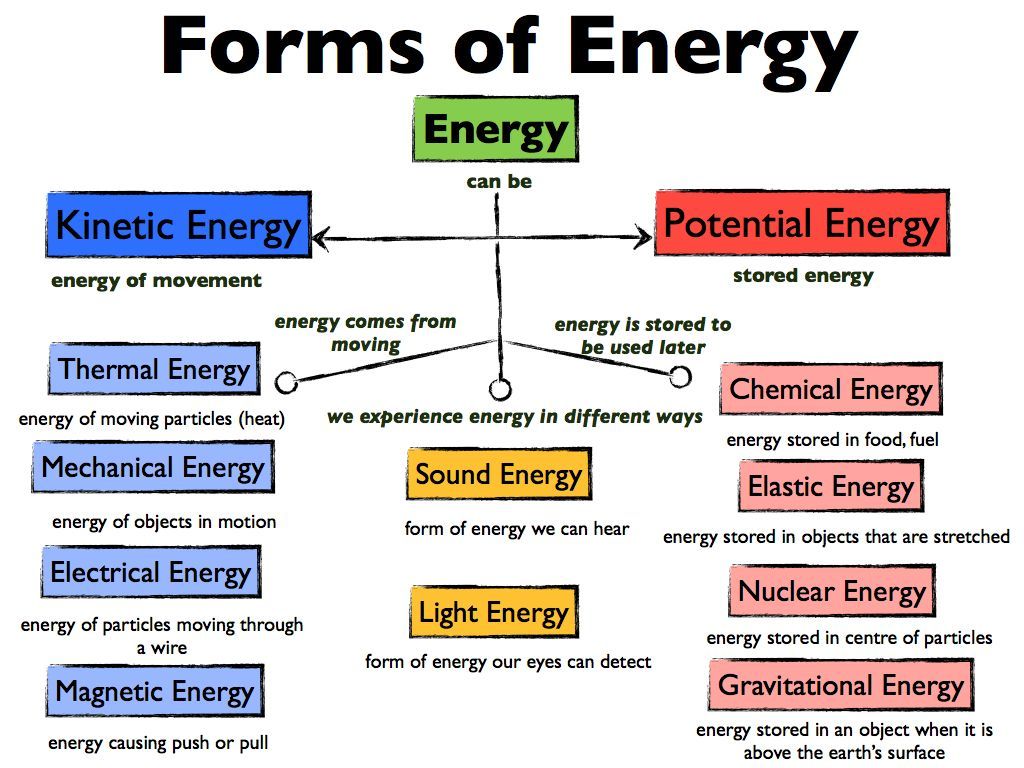 Learning Goal We Are Learning To Explain The Different Forms Of Energy We Encounter Science Lessons Physical Science Experiments Physical Science High School
Learning Goal We Are Learning To Explain The Different Forms Of Energy We Encounter Science Lessons Physical Science Experiments Physical Science High School
 Related Image Free Energy Free Energy Projects Free Energy Generator
Related Image Free Energy Free Energy Projects Free Energy Generator
 The Laws Of Thermodynamics Thermodynamics Chemistry Education Engineering Science
The Laws Of Thermodynamics Thermodynamics Chemistry Education Engineering Science
 Ionization Energy Definition Ionization Energy How To Increase Energy How To Remove
Ionization Energy Definition Ionization Energy How To Increase Energy How To Remove
 Enthalpy Gif Gif Image 621 466 Pixels Physics And Mathematics Science Chemistry Physics Books
Enthalpy Gif Gif Image 621 466 Pixels Physics And Mathematics Science Chemistry Physics Books
 Enthalpy Physics And Mathematics Thermodynamics Engineering Science
Enthalpy Physics And Mathematics Thermodynamics Engineering Science
Post a Comment for "Definition Of Free Energy In Chemistry"