What Are The Energy Levels Of An Atom Occupied By
The energy levels of an atom are occupied by ____. The first energy level can contain 2 1 2 or two electrons.
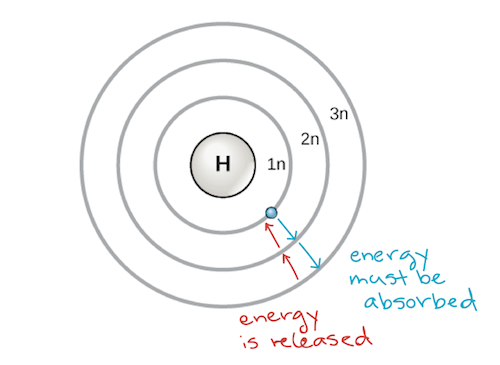
Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus.

What are the energy levels of an atom occupied by. The electrons surrounding an atom are located in regions around the nucleus called energy levels. The first energy level is closest to the nucleus. The range of the quantum number can fluctuate between the lowest energy level to the highest energy level.
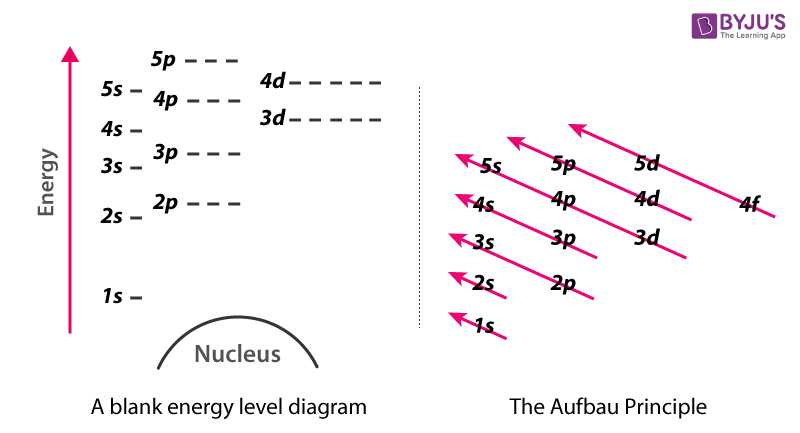
When we move across a period from left to right in a periodic table the number of electrons in atoms increases within the same orbit. Energy is quantized so the higher the principal quantum number n usually the higher the energy of the orbital. The electron configuration of an atom in the ground state is 1s2 2s2 2p2.
An electron dot diagram shows _____. The second energy level is a. The first principal energy level has one sublevel that contains one orbital called the s orbital.
The energy levels of an atom are occupied by ___. Kvargli6h and 10 more users found this answer helpful. The total number of occupied energy levels in this atom is A 1 B 2 C 3 D 4.
The period number that an element is in is the number of energy levels that the element has. These numbers are quantum numbers. However according to something called the Pauli exclusion principle a result of quantum mechanics each allowed energy level can be occupied by no more than two electrons of opposite spin.
Oct 17 2016. This just means that at low temperatures all available states in the crystal up to a certain energy level will be occupied by two electrons 1. An orbital at the frontier of chemical reactions performing the interesting legwork to move the reaction forward.
In the compound H2O the electrons in the bonds are unequally shared between O and H forming ____. Energy levels inside an atom are the specific energies that electrons can have when occupying specific orbitals. All the energy levels are denoted by integers eg.
The period number denoted by n is the outer energy level that is occupied by electrons in an atom. The energy levels of an atom are occupied by electrons D. Ordering orbitals by energy is straightforward.
Group 1 will have 1 valence electron while Group 2 will have 2 valence electrons. Shown here is the first Balmer transition in which an electron jumps from orbit n 3 to orbit n 2 producing a photon of red light with an energy of 189 eV and a wavelength of 656 nanometres. How many electrons are needed in the outer energy levels of most atoms for the atom to be chemically stable.
An atom has 3 valence electrons and 4 occupied main energy levels The Periodic Table In a previous section the periodic table was introduced as a list of the elements. N1 or 2 3 and so on. We also pointed out that the design of the periodic table separates the metals from the nonmetals.
Those are your valence electrons. Electrons can be excited to higher energy levels by absorbing energy from the surroundings. Light is emitted when an electron relaxes from a high energy state to a lower one.
An energy level represents the 3-dimensional space surrounding the nucleus where electrons are most likely to be. They consist of the highest energy level electrons of any atom. The maximum number of electrons in the second energy level of an atom is ____.
It is the highest-energy atomic orbital in an atom that is filled with electrons. The second can contain up to 2 2 2 or eight electrons. Group 13 will have 3 Group 14 will have 4 and so on.
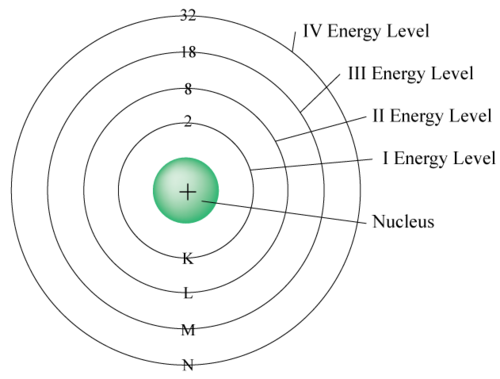
The third can contain up to 2 3 2 or 18 electrons and so on. The first energy level K contains 2 electrons second energy level 8 third energy level 18 and fourth energy level has 32 electrons. 116 rows Number of Electrons in each Level.
The energy levels of an atom are occupied by ____. It is otherwise known as a valence orbital or a frontier orbital ie.
 Energy Level Definition Equation W Diagrams
Energy Level Definition Equation W Diagrams
 Atom Orbits And Energy Levels Britannica
Atom Orbits And Energy Levels Britannica
 How To Represent Electrons In An Energy Level Diagram Dummies
How To Represent Electrons In An Energy Level Diagram Dummies
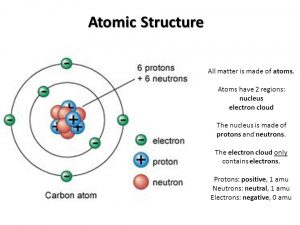 Atomic Structure Of Matter Energy Levels Electronic Distribution Chemical Activity Science Online
Atomic Structure Of Matter Energy Levels Electronic Distribution Chemical Activity Science Online
 Atomic Energy Levels Video Khan Academy
Atomic Energy Levels Video Khan Academy
Chemistry I Atoms And Molecules
 Chemical Bonding Biology For Non Majors I
Chemical Bonding Biology For Non Majors I
 Atomic Structure Nucleus Proton Neutron Electron Mass Charge Isotopes Electron Arrangement Rutherford Bohr Model Of Atom Allotropes History Of Atomic Structure Model Development Ionisation Ions Gcse Chemistry Revision Notes Quizzes Ks4 Science
Atomic Structure Nucleus Proton Neutron Electron Mass Charge Isotopes Electron Arrangement Rutherford Bohr Model Of Atom Allotropes History Of Atomic Structure Model Development Ionisation Ions Gcse Chemistry Revision Notes Quizzes Ks4 Science
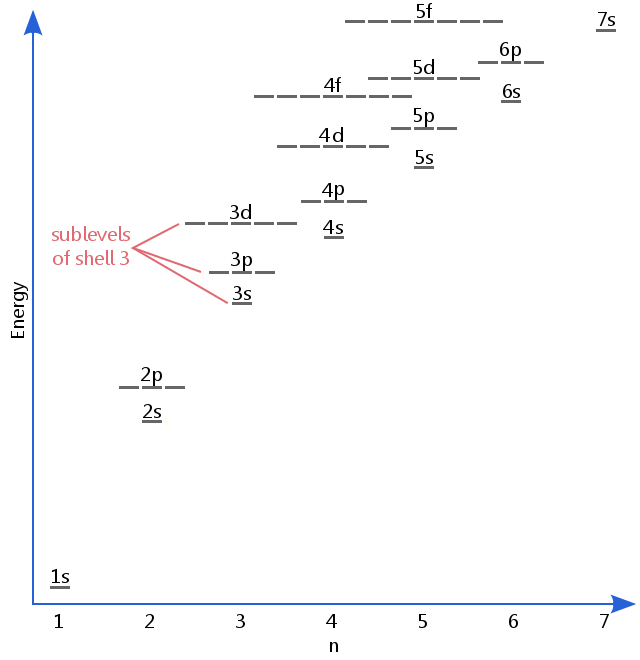 Definition Of Sublevel Chemistry Dictionary
Definition Of Sublevel Chemistry Dictionary
 Atom Orbits And Energy Levels Britannica
Atom Orbits And Energy Levels Britannica
 Energy Level Diagram Different Energy Shells Around The Nucleus
Energy Level Diagram Different Energy Shells Around The Nucleus
 Electron Configuration Boundless Chemistry
Electron Configuration Boundless Chemistry
The Periodic Table By Energy Levels
 Atom Orbits And Energy Levels Britannica
Atom Orbits And Energy Levels Britannica
 Energy Level Diagram Different Energy Shells Around The Nucleus
Energy Level Diagram Different Energy Shells Around The Nucleus
 4 8 Electrons Chemistry Libretexts
4 8 Electrons Chemistry Libretexts

Post a Comment for "What Are The Energy Levels Of An Atom Occupied By"