What Is The Equipartition Theorem Used For
What does EQUIPARTITION THEOREM mean. Can somebody please explain how it works.
 Solved The Equipartition Theorem Consider The Potential Chegg Com
Solved The Equipartition Theorem Consider The Potential Chegg Com
The equipartition theorem is a principle of classical non- quantum statistical mechanics which states that the internal energy of a system composed of a large number of particles at thermal equilibrium will distribute itself evenly among each of the quadratic degrees of.

What is the equipartition theorem used for. The equipartition theorem the energy is shared out evenly amongst the x y and z translational degrees of freedom. Contributors and Attributions. The additional energy obtained by gas molecules is not evenly distributed for each degree of freedom but is divided gradually.
The equipartition result serves well in the definition of kinetic temperaturesince that involves just the translational degrees of freedom but it fails to predict the specific heatsof polyatomic gases because the increase in internal energy associated with heating such gases adds energy to rotational and perhaps vibrational degrees of freedom. What does Equipartition theorem say about energy distribution. Sep 10 2009 1 jaejoon89.
It states that the mean value of every independent quadratic term in the energy is equal to. The equipartition theorem can go further than simply predicting that the available energy will be shared evenly amongst the accessible modes of motion and can make quantitative predictions about. How do you use the equipartition theorem to calculate molar specific heat for hydrogen gas.
Well the equipartition theorem for the free particle at high enough temperatures is. Imagine a hollow container evacuated except for two lonely molecules of equal mass a practical impossibility of course but a useful idealization for purposes of discussion. Here we mention only that in a system the energy is.
The equipartition theorem in turn is a theoretical result derived from the ideas of mechanics and the ideas of probability and statistics applied to molecular collisions. I dont know what degree of freedom means for instance. EQUIPARTITION THEOREM meaning - EQUIPARTITION TH.
Equipartition theorem Thread starter jaejoon89. This phenomenon is known as the equipartition of energy theorem. The energy equipartition theorem states that total energy must be divided equally for each degree of freedom.
The equipartition theorem is a statement from statistical physics and states that in equilibrium for every generalized momentum or generalized coordinate acting in the formula for the energy of the system quadraticaly there is a mean energy of frac12kT where kis the Boltzmann constant and Tthermodynamic temperature of the system. Start date Sep 10 2009. It says that if you have a container of gas molecules the internal energy of the system will be divided equally between the different quadratic squared degrees of freedom available.
The equipartition theorem can be used to derive the Langevin equation. Each atom in such a solid can oscillate in three independent directions so the solid can be viewed as a system of 3Nindependent simple harmonic oscillators where N. The equipartition theorem can be used to derive the classical ideal gas law and the DulongPetit law for the specific heat capacities of solids.
This is the famous equipartition theorem of classical physics. It can also be used to predict the properties of stars even white dwarfs and neutron stars since it holds even when relativistic effects are considered. The best way to track these modes is to imagine energy going into one of them at a time without changing the energy in the previous ones.
This fact follows from a more general result the equipartition theorem which holds in classical non-quantum thermodynamics for systems in thermal equilibrium under technical conditions that are beyond our scope. Bb E_ avg K_ avg N2nRT. The equipartition theorem also known as the law of equipartition equipartition of energy or simply equipartition states that every degree of freedom that appears only quadratically in the total energy has an average energy of k B T in thermal equilibrium and contributes k B to the systems heat capacity.
If all terms in the energy are quadratic then the mean energy is spread equally over all degrees of freedom hence the name equipartition. An important application of the equipartition theorem is to the specific heat capacity of a crystalline solid. Where Frndis a random force representing the random collisions of the particle and the surrounding molecules and where the.
So for example a particle can be moving in the x direction only and it has a kinetic energy.

Validity Of Equipartition Theorem Physics Forums
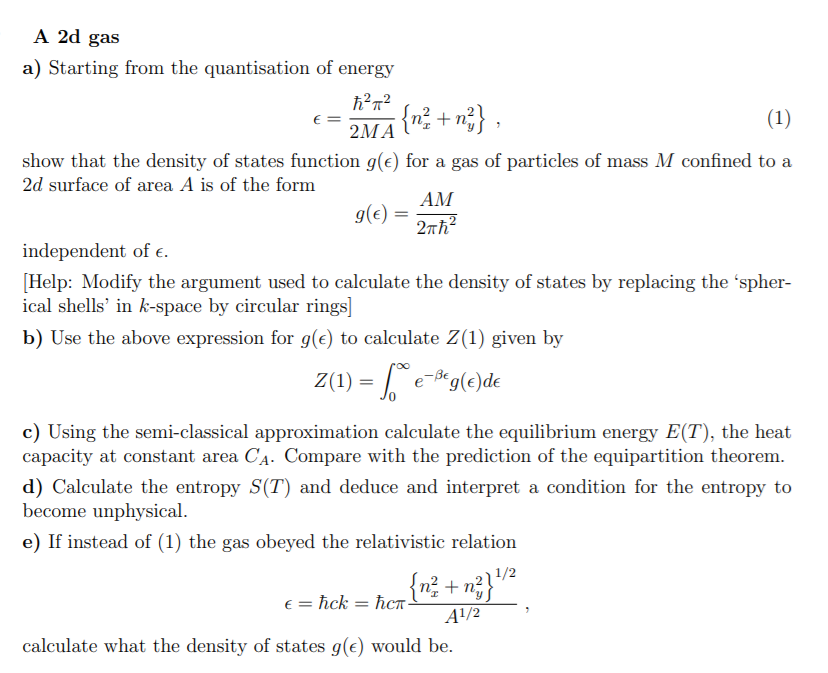 Solved Questions C D E Please Explain The Last Part Chegg Com
Solved Questions C D E Please Explain The Last Part Chegg Com
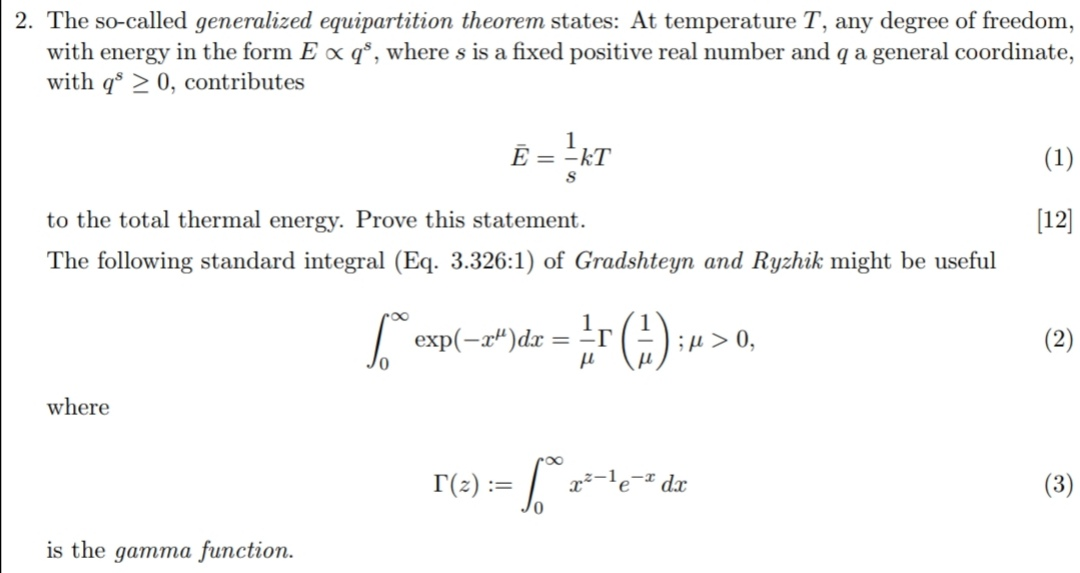 2 The So Called Generalized Equipartition Theorem Chegg Com
2 The So Called Generalized Equipartition Theorem Chegg Com
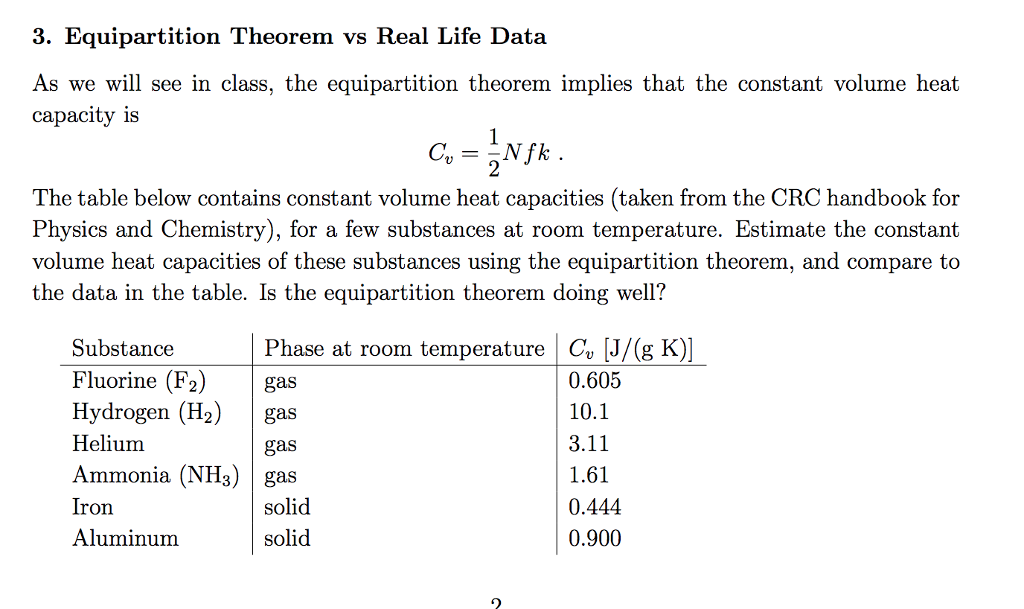 Solved 3 Equipartition Theorem Vs Real Life Data As We W Chegg Com
Solved 3 Equipartition Theorem Vs Real Life Data As We W Chegg Com
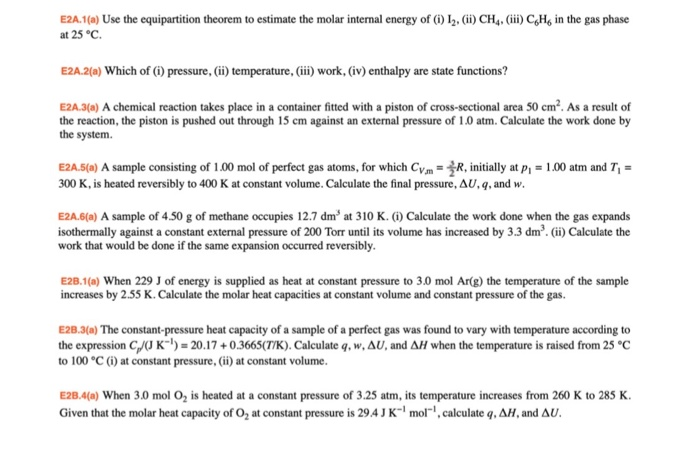 Solved E2a 1 A Use The Equipartition Theorem To Estimate Chegg Com
Solved E2a 1 A Use The Equipartition Theorem To Estimate Chegg Com
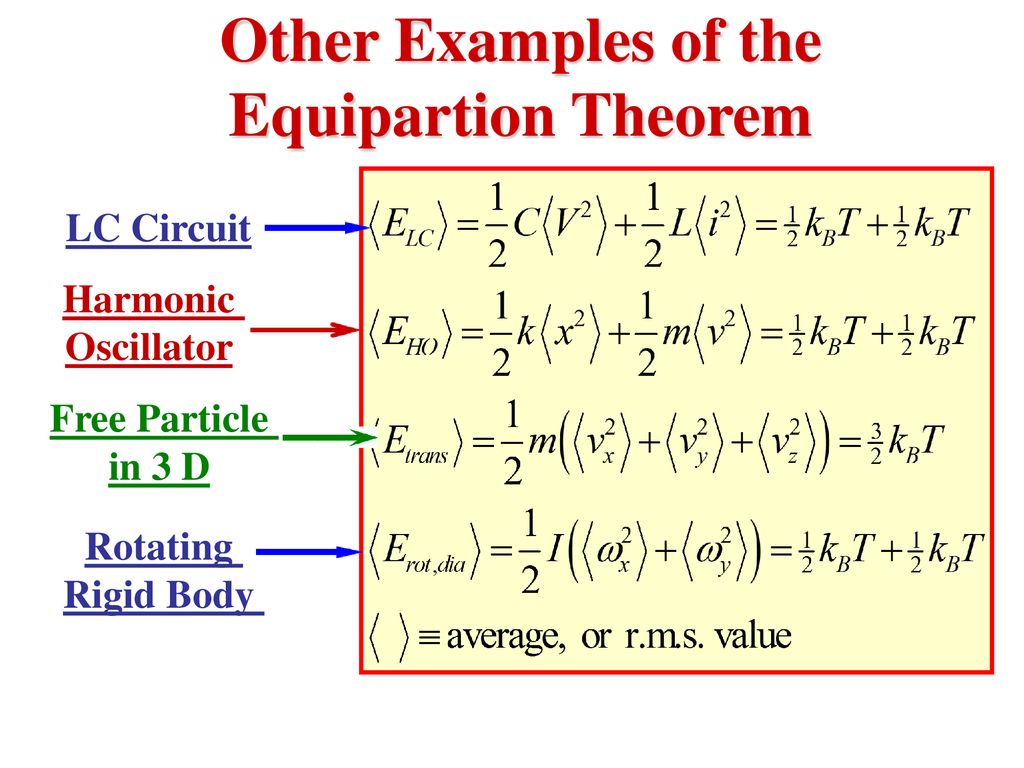 Recall The Equipartition Theorem In Ch 6 Ppt Download
Recall The Equipartition Theorem In Ch 6 Ppt Download
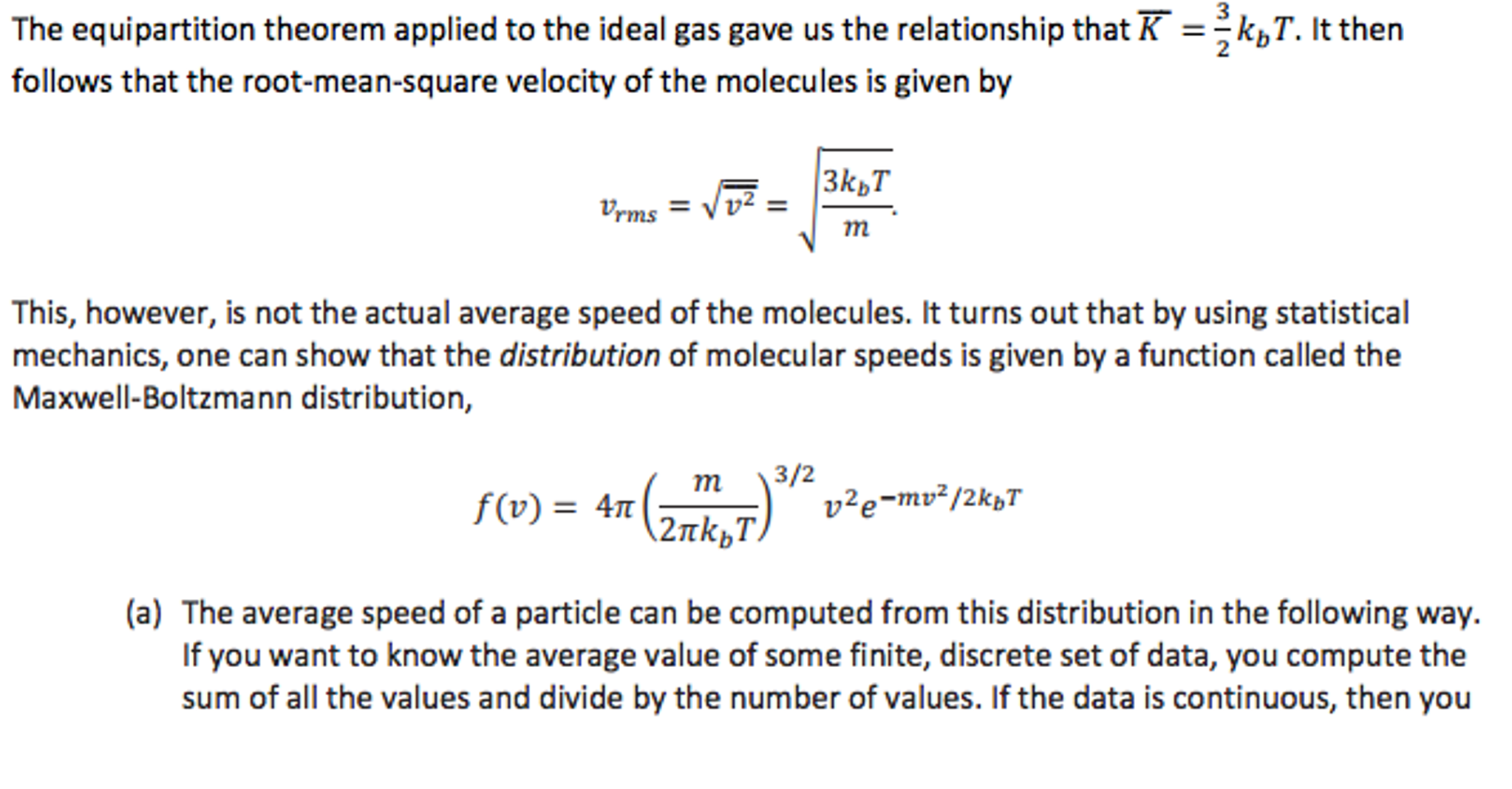 Solved The Equipartition Theorem Applied To The Ideal Gas Chegg Com
Solved The Equipartition Theorem Applied To The Ideal Gas Chegg Com
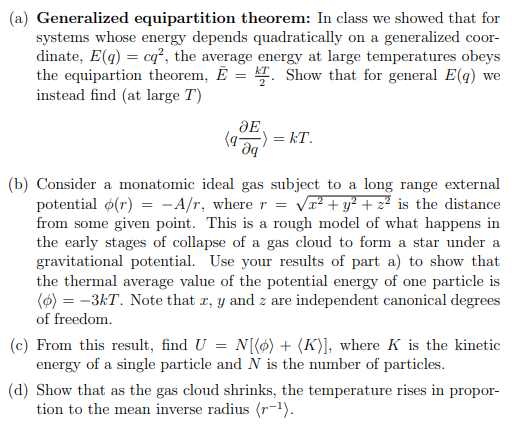 Solved A Generalized Equipartition Theorem In Class We Chegg Com
Solved A Generalized Equipartition Theorem In Class We Chegg Com
 Ppt 9 1 Historical Overview 9 2 Maxwell Velocity Distribution 9 3 Equipartition Theorem Powerpoint Presentation Id 6980923
Ppt 9 1 Historical Overview 9 2 Maxwell Velocity Distribution 9 3 Equipartition Theorem Powerpoint Presentation Id 6980923
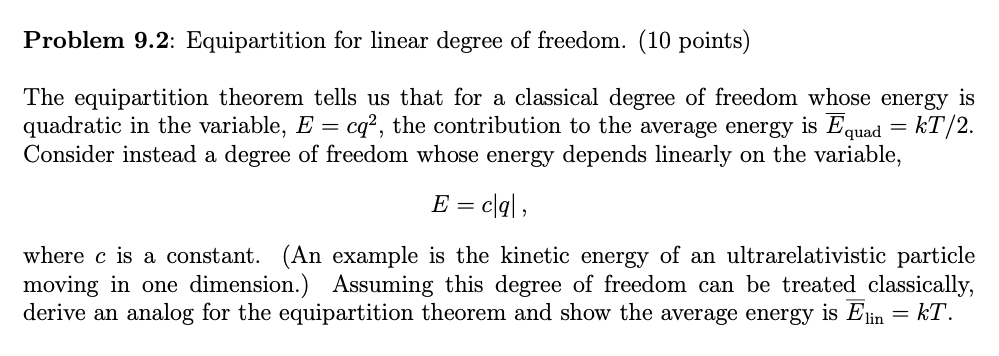 Solved Problem 9 2 Equipartition For Linear Degree Of Fr Chegg Com
Solved Problem 9 2 Equipartition For Linear Degree Of Fr Chegg Com
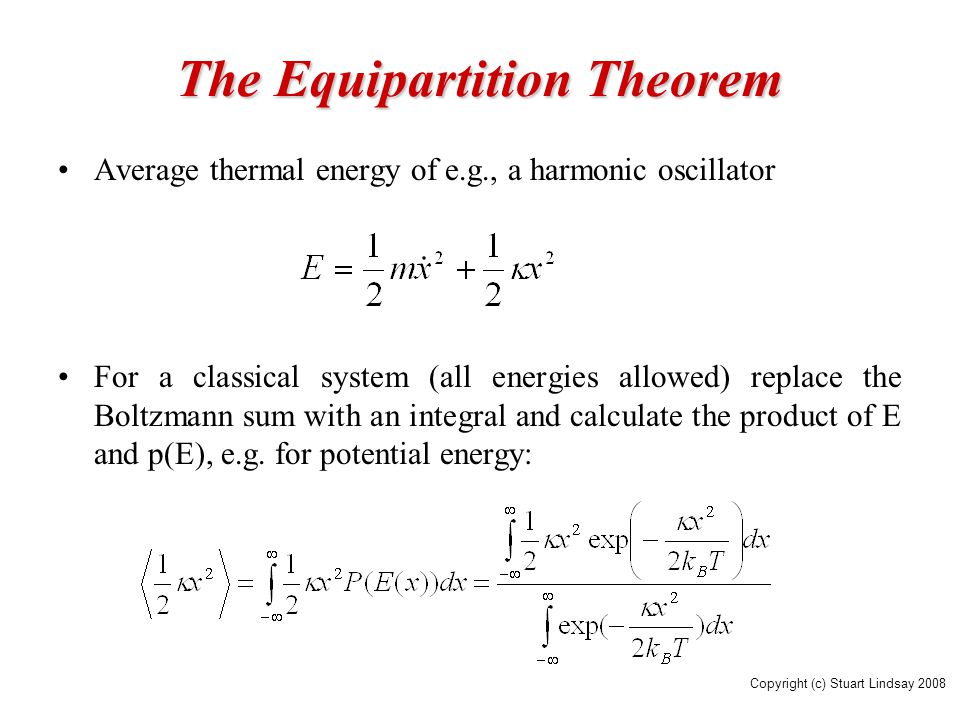 Statistical Mechanics Ppt Video Online Download
Statistical Mechanics Ppt Video Online Download
 Solved The Equipartition Theorem Applied To The Ideal Gas Chegg Com
Solved The Equipartition Theorem Applied To The Ideal Gas Chegg Com
 Solved The Equipartition Theorem Consider The Potential Chegg Com
Solved The Equipartition Theorem Consider The Potential Chegg Com
Https People Nscl Msu Edu Witek Classes Phy321 Energy Energy2 Pdf
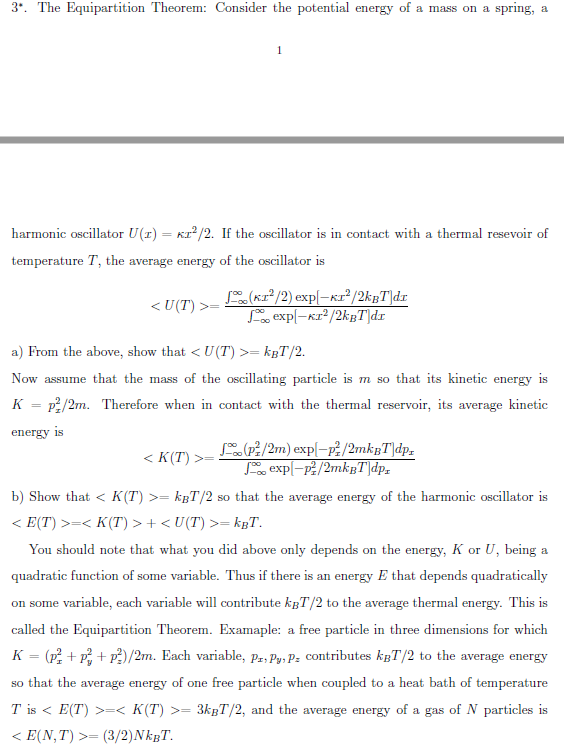 Solved The Equipartition Theorem Consider The Potential Chegg Com
Solved The Equipartition Theorem Consider The Potential Chegg Com
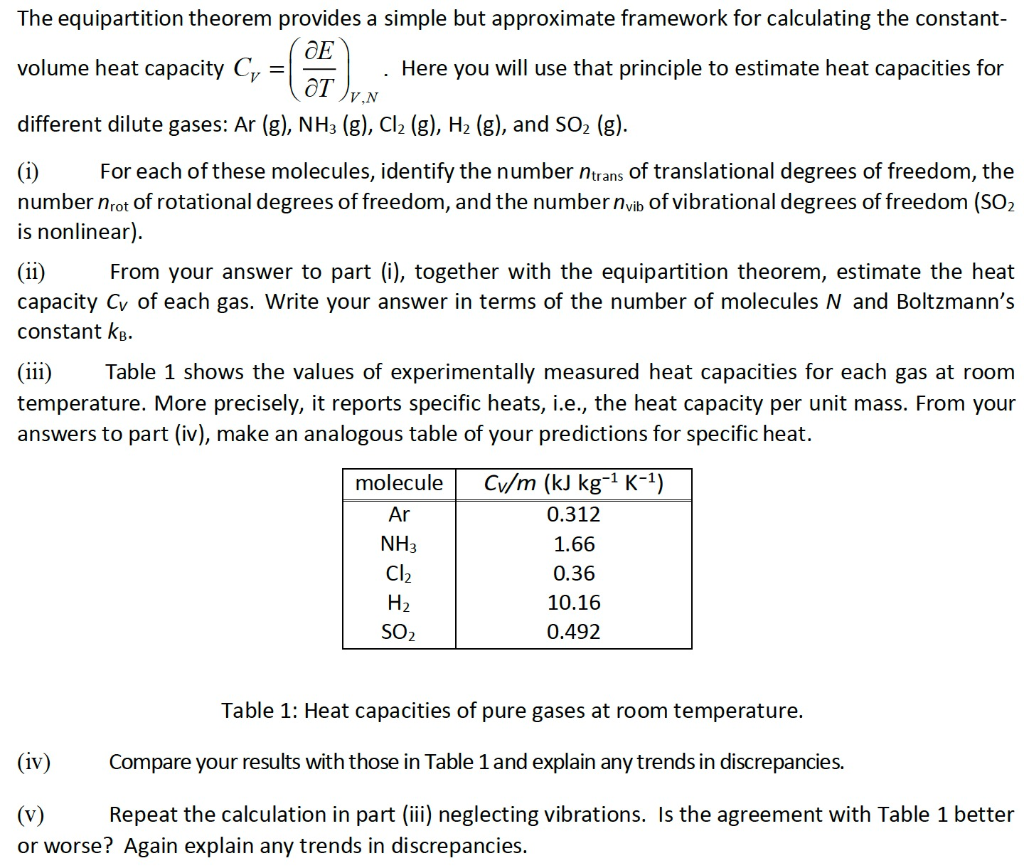 Solved Capacity C Ce The Equipartition Theorem Provide Chegg Com
Solved Capacity C Ce The Equipartition Theorem Provide Chegg Com
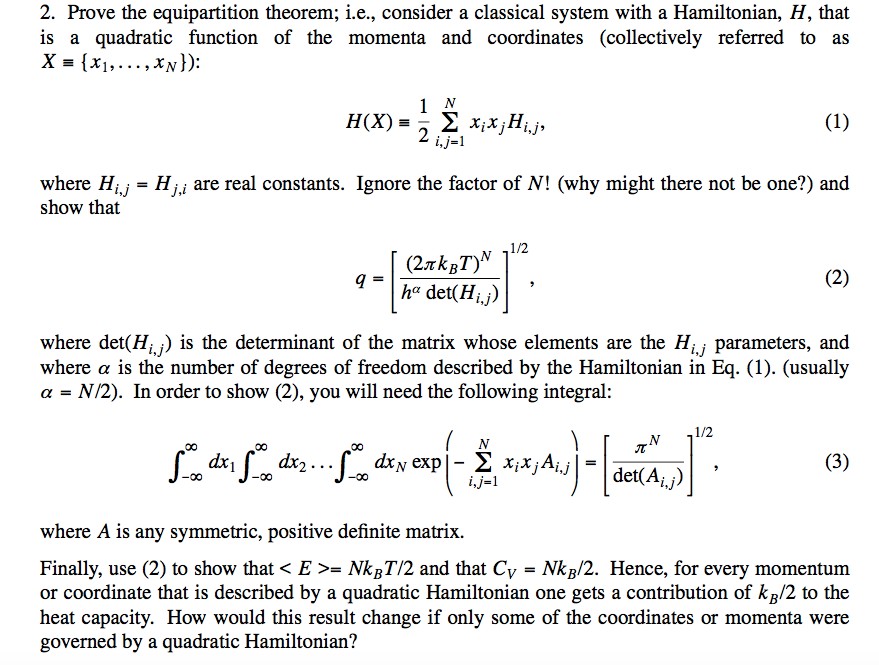

Post a Comment for "What Is The Equipartition Theorem Used For"